It is possible to derive oleacein from olive leaves transforming theoleuropein (present as Glucosidic secoiridoid) through the use of a recyclable acid catalyst, proton exchange montmorillonite (H-mont) [1].
La montmorillonite it is a clay composed of aluminum and magnesium phyllosilicate whose name derives from the locality of Montmorillon, in France, where it was found for the first time. Chemically, it is a hydrated sodium, calcium, aluminum and magnesium silicate hydroxide. Montmorillonite clays have been widely used, for over 60 years, in catalytic processes, as catalysts of cracking. Other acid-based catalysts use them after treating them with an acid [2].
Its crystals are characterized by a crystalline structure, formed by overlapping sheets of pyrophyllite, with intercalated water molecules, coordinated by sodium ion (Na+); the peculiarity is that this ion represents the so-called “exchangeable ion”, being able to be easily replaced by others, in our case hydrogenions (H+).
I remember that, in chemistry, an acid is a molecule that can release protons (H+) therefore by treating montmorillonite with a strong acid we can replace its sodium ions (Na+) with hydrogen ions (also called "protons" H+ as they are hydrogen atoms that have lost the only electron and therefore the charge of the atom , which remains, is the positive one of the only proton of the nucleus).
While the conventional chemical synthesis of oleacein, if performed starting from D-lyxose, would require more than 10 steps and would lead to a final yield of 13%, however, if oleuropein was used, which we find abundantly in olive leaves, it would have yields above 80%.
Montmorillonite plays a rolethe catalyst solid with “acid” function (H-mont) can also be easily recovered and reused at least five times, through simple cleaning.
Importantly, this synthesis procedure is Also applicable to other glucosidic secoiridoidsi, furthermore it can also be used for the corresponding “scale-up” reaction using oleuropein extracted from olive leaves as starting material.
This reaction is also applicable to the synthesis of a compound analogous to oleacein, such asoleocanthal, in this case it would be obtainable starting from ligstroside, also contained in olive leaves.
Due to the rare presence of oleacein in olive oil, its biological functions have, over time, been studied to a much lesser extent than those of oleocanthal. Nonetheless, research has shown that theoleacein owns a business antioxidant, inflammatory, an activity inhibitory towards the enzymes of conversion of angiotensin related to processes hypertension, (i.e. it is equipped with an ACE-inhibitory function and took its term from this activity “oleacein”, coined in 1996 by Danish K. Hansen.
This denomination is derived from a merger between “olea” “ACE” “Inhibitor”. The acronym “ACE” is going to “Angiotensin–Converting Enzyme”, or the enzyme convertingangiotensin I in angiotensin II. Only this last molecule is capable of releasing aldosterone from the adrenal glands, with the aim of increasing blood pressure) [3].
Furthermore, oleacein has protective effects on damage / metabolic alterations caused by a high-fat diet, has a antitumor activity in multiple myeloma. Finally it can increase the level of adenosine triphosphate (ATP), in a cellular model of Alzheimer's disease, but only in the initial phase.
As a preliminary experiment, performed by Shimamoto's group, it was the realization of a hydrolysis of oleuropein in the presence of hydrochloric acid and it was seen that the oleacein yield increased with decreasing acid concentration himself, arriving at a surrender of 67%.
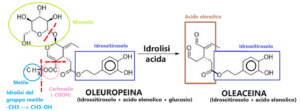
But the acidic reaction allowed for simultaneous hydrolysis of the methyl ester and decarboxylation to give the demethylated and decarboxylated oleacein molecule (see attached diagram). Notably, only a small amount of acid was needed for this reaction.
It has also been used, instead of hydrochloric acid,p -toluenesulfonic acid, achieving, in this case, a surrender of 77% as the amount of acid decreased. This suggested using a solid acid and, among the many tried, the choice was for proton exchange montmorillonite, pretreated with hydrochloric acid, which allowed to obtain yields above 80% (as already mentioned, the sodium ion Na+ in the clay was replaced, in the pre-treatment, with the acid ion – the proton H+)
Oleuropein has two ester groups, the methyl ester and the hydroxytyrosol ester, which may be involved in the synthesis of oleacein from oleuropein. However, only the methyl group needs to be hydrolyzed and decarboxylated to give oleacein (see attached diagram).
The results of this study suggested that the hydrolysis of the methyl ester in oleuropein, is more favorable than the hydrolysis of the hydroxytyrosol group (the activation energies for the hydrolysis of the methyl ester were 81,12 kJ mol −1).
To demonstrate an application of this catalysis, Shimamoto attempted to synthesize l'oleocanthal from ligstroside (as a glucosidic secoiridoid) using H-mont as a solid acid catalyst. In this case the oleocanthal was formed with a yield of 63%, when the reaction was carried out at elevated temperatures for 12 hours. Therefore, the development of an efficient process to synthesize oleacein from oleuropein may allow us to discover potential functions implicit in oleacein itself, which to date elude us.
Furthermore, since oleuropein is abundantly present in the leaves, this strategy will guarantee the efficient use of olive tree waste even in the field nutraceutical, as a property straddling the “nutritional” and the activity “pharmaceuticals”.
REFERENCES
[1] Lloyd Lawrie 2011. Handbook of Industrial Catalysts. New York: Springer. pp. 181–82. ISBN 978-0387246826.
[2] Shimamoto Y. et al. 2023. Solid acid-catalyzed one-step synthesis of oleacein from oleuropein. SciRep.; 13:8275.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10203140/
[3] Hansen K., et al. 1996. Isolation of an Angiotensin Converting Enzyme (ACE) Inhibitor from Olea europaea and Olea lancea. Phytomedicine.; 2:319–325. doi: 10.1016/S0944-7113(96)80076-6.
Browse for free l'Olivo News click , here



















